Lungscreen is the only advanced, integrated, and comprehensive lung disease diagnostic, assessment, reporting and data storage (VNA) solution in Australia. We purpose built our organisation as a dedicated lung screening service to deliver the best patient outcomes.
Our service model was designed from the ground up and is based on over 30+ years of experience in lung diagnostics. Lungscreen's service model is many steps ahead of the industry's traditional radiology service model.
Our service model is designed to provide a complete lung screening program for individuals at risk, referring physicians, radiologists, and administrators. We created a reporting, data and patient registry system that can collect, display, and share very granular data from other providers into Lungscreen's Vendor Neutral Archive System (VNA).
We believe a collaborative approach is the key to delivering a quality screening program. Achieving this requires a united vision, innovation, and shared responsibility between health professionals to achieve the best health outcome for the patient. Our model fosters a culture of collaboration and education between radiologists, resulting in a broader pool of radiologists with advanced expertise and qualifications in lungs, as well as increased knowledge and higher engagement across the industry.
Put simply, Lungscreen leverages the experience and ongoing training of our highly skilled team to provide a superior lung screening service to patients that cannot be achieved by a more fragmented, traditional approach to screening.
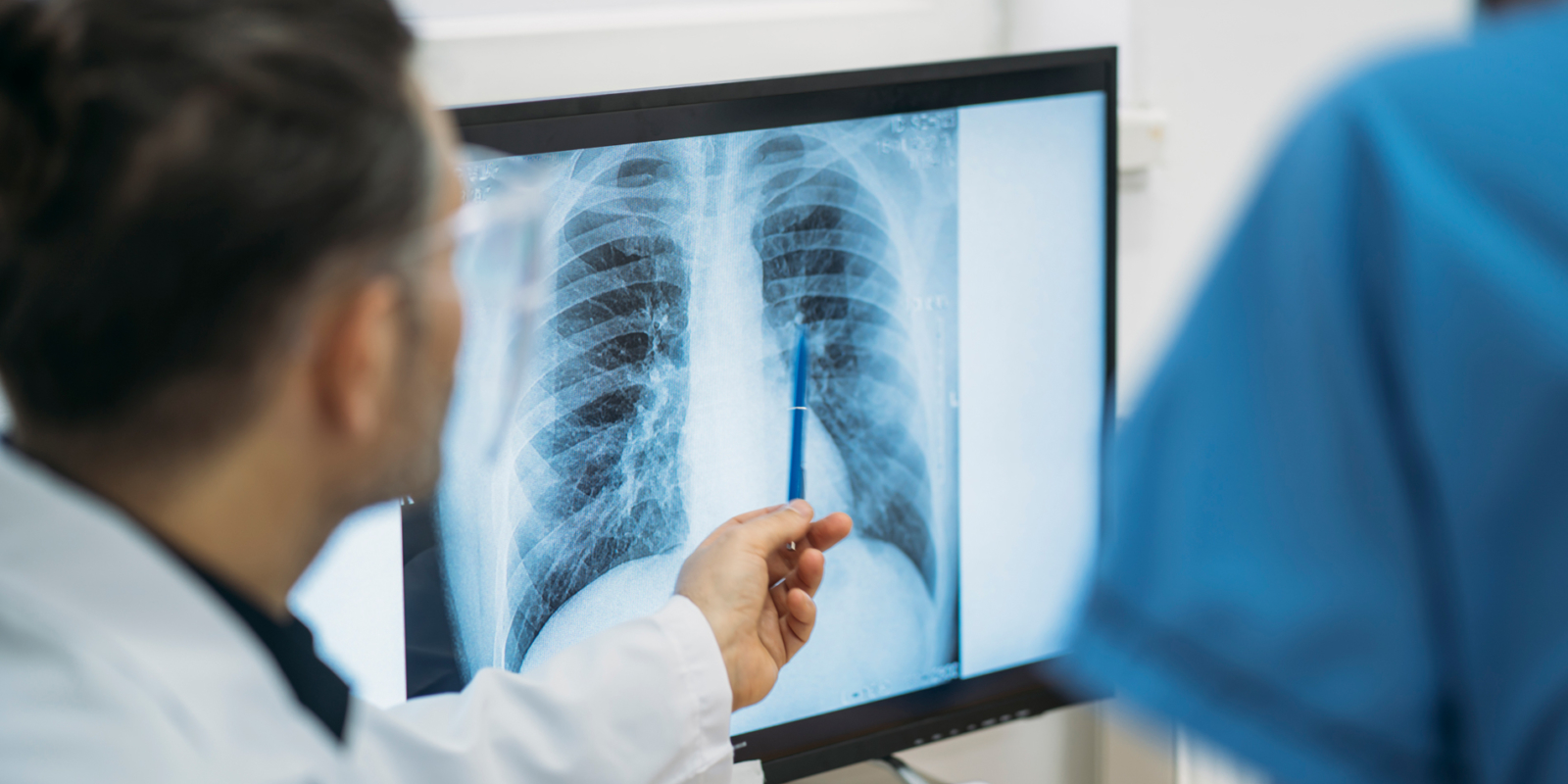
Lungscreen has a rigorous quality assurance focus both internally and with our partnered radiology providers. Lungscreen is benchmarked internationally, engaging with the recognised leaders in ILO reporting, the University of Illinois at Chicago (UIC), in an auditing program. Lungscreen provides constant feedback on chest x-ray image quality and facilitates webinar training with our radiology partners. Ensuring image quality standards are met in accordance with ILO classifications.
Lungscreen Australia is an ISO 9001:2015 certified organisation. This certification is the most established international standard for Quality Management systems. The ISO certification recognises our commitment to excellence that is built around our quality service, processes, continuous improvement, and risk management.
Our ISO quality management system ensures quality is consistently achieved both internally and externally in accordance with AS/NZS ISO 9001:2015
Internally, it guarantees our processes align with global standards and ensures Lungscreen is stable and ready for growth.
Externally, it gives our stakeholders confidence in our quality of service and credibility within the industry.
Lungscreen has invested in a state-of-the-art Vendor Neutral Archive (VNA) hosted on Microsoft Azure Servers based in Australia. Microsoft Azure is endorsed by the Australian Federal Government due to its security protocols. This ensures that all data is secure in line with Australia's privacy policies. Furthermore, Lungscreen's VNA is scalable, providing the capacity required to support further demand.
Lungscreen has developed its own cloud-based reporting software. This software gives radiologists access to template reporting and ensures all radiologists follow the same process of reporting. This also enables our radiologists to access cases securely on the cloud and report anytime and from home. All radiologists are provided with a high resolution, fully secure workstation supplied and managed by Lungscreen's IT personal. This is to ensure high quality and compliance with the recommendation and accreditation standards.
Lungscreen utilises Artificial Intelligence technology to increase the sensitivity and accuracy of our reporting. All cases are reviewed by AI and presented to our radiologists for reporting. The AI is used as a tool to assist in the detection and description of the lesions where needed. In addition, all other incidental findings, including vascular and heart pathologies, are reported for clinical review.